Graphyne
Scientists have finally developed graphyne, a new amazing substance.Scientists have been attempting to synthesise an unique type of carbon called graphyne for over a decade with too little success. However, University of Colorado Boulder researchers have finally succeeded in producing the elusive carbon allotrope.
What is Graphyne?
Graphyne is a carbon allotrope. Its crystal lattice is made up of one-atom-thick planar sheets of sp and sp2-bonded carbon atoms. A lattice of benzene rings joined by acetylene bonds can be seen.
Is Graphyne stronger than graphene? Graphyne vs graphene
Because of its triple bonds, graphyne is predicted to be more stiff than graphene. Because of its greater stiffness, graphyne is likely to be less flexible, brittle, and mechanically weaker than graphene.
What is structure of Graphyne?
Graphyne has yet to be synthesised in sufficient amounts for research, but scientists have been able to anticipate certain features of the material based on expected lattice geometries using computer models. The suggested graphyne structures are created by substituting acetylene bonds for carbon-carbon single bonds in a graphene lattice.
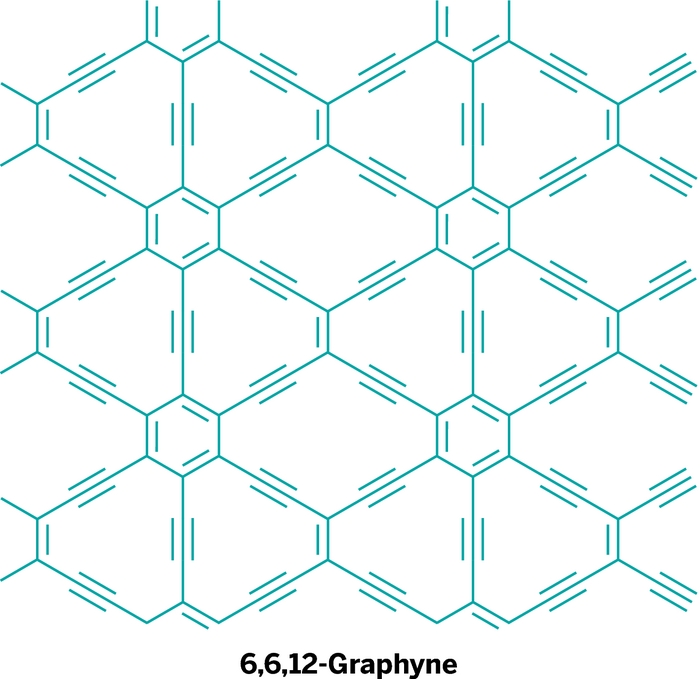
Graphyne is thought to occur in a variety of shapes. The different configurations of sp and sp2 hybridised carbon account for this variation. A hexagonal lattice structure and a rectangular lattice structure are among the potential geometries. Due to the possibility of direction-dependent Dirac cones, it has been proposed as being preferable to graphene for some uses. The rectangular lattice of 6,6,12-graphyne may have the highest promise for future uses of the postulated structures.
Properties of Graphyne
The models for graphyne demonstrate that its double and triple linked carbon atoms have the potential for Dirac cones. There is a single location in the Fermi level where the conduction and valence bands meet in a linear pattern due to the Dirac cones. The benefit of this approach is that electrons behave as if they had no mass, resulting in energies proportional to electron momentum. Hexagonal graphyne, like graphene, possesses direction-independent electric characteristics.
However, the electric characteristics of the suggested rectangular 6,6,12-graphyne would fluctuate in various orientations in the plane of the material because to its symmetry. The symmetry of graphyne allows it to self-dope, which means it has two distinct Dirac cones slightly above and below the Fermi level. Applying in-plane external strain can effectively tune the self-doping effect of 6,6,12-graphyne. To date, graphyne samples have showed a melting point of 250-300 °C, as well as minimal reactivity in breakdown reactions with oxygen, heat, and light.
Posted By InnoTechzz